
What is hard water?
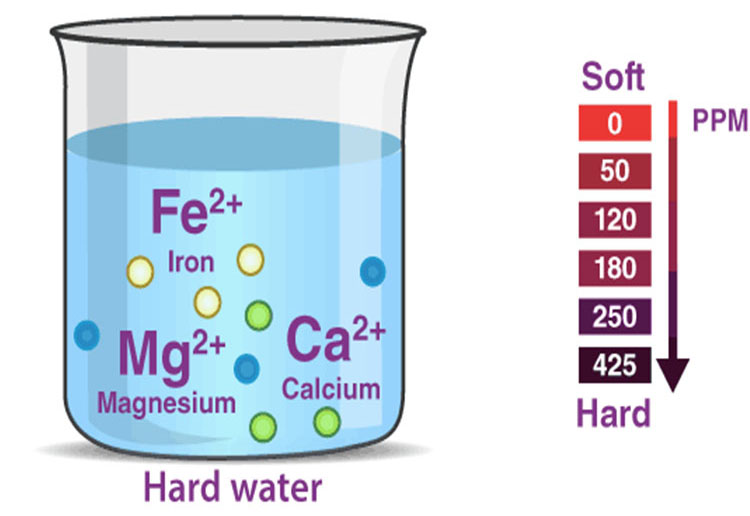
Hard water refers to water that contains more soluble calcium and magnesium compounds.
The hardness of water refers to the content of salts dissolved in water, that is, the content of calcium salts and magnesium salts. The higher the content, the higher the hardness, and vice versa. GPG is the unit of water hardness, 1GPG means that the content of hardness ions (calcium and magnesium ions) in 1 gallon of water is 1 grain. According to the American WQA (Water Quality Association) standard, the hardness of water is divided into 6 grades: 0~0.5GPG is soft water, 0.5~3.5GPG is slightly hard, 3.5~7.0GPG is medium hard, 7~10.5GPG is hard water, 10.5~ 14.0GPG is very hard, and above 14.0GPG is extremely hard.
Hard water does not pose a direct health hazard, but it can bring a lot of trouble to life, such as scale buildup on water appliances, and reduced washing efficiency with soaps and detergents.
Origin
When water condenses in the atmosphere, it reacts with carbon dioxide in the air to form a weak acid called carbonic acid. This acid eventually falls to the ground with the rain, then flows over the upper part of the soil to the rock layer, where the carbonic acid dissolves the lime (calcium carbonate and magnesium carbonate), neutralizes it, and hardens at the same time. This is how the karst caves in some areas and the hard water near the karst caves are formed. Hard water can be divided into temporary and permanent. Temporary hard water is usually related to calcium and magnesium carbonate and bicarbonate. Such crystals can exist in the water for a long time until the pressure or temperature changes, making the water supersaturated. Causes deposits to adhere to hot or rough surfaces, such as in pipes and heat exchangers, forming hard scale; permanent hard water is mainly related to calcium sulfate and magnesium sulfate, and is not affected by heat and air pressure changes, but such as As the water evaporates, hard limescale remains and forms.
Industrial hard water is an industrial term for whether calcium, magnesium and other minerals in the pipeline will be deposited. It has been used as one of the judgment criteria for production cost.

Character
Water is a good solvent and can effectively remove dirt impurities. Pure water - colorless, tasteless, and odorless, it is called a "universal solvent". When the specific solvent is not mentioned, the solvent will be defaulted to water. Water dissolves better when water and carbon dioxide combine to produce trace amounts of carbonic acid. When water flows over land and rocks, it dissolves small amounts of mineral components, two of the most common of which are calcium and magnesium, i.e. they harden the water. The more minerals such as calcium and magnesium in the water, the harder the water is. Even in hard water areas, as long as the saturation index of the water is not exceeded, scale will form in the water pipes. If it is below the saturation index or just at the critical point of saturation, there will be no scale formation. Conversely, scale will form in soft water if the saturation index is exceeded.
The saturation index is determined by the pH of the water. As we all know, pH is determined by the pH test:
The lower the pH value, the stronger the acidity of the water, the higher the saturation index, and the more minerals that can be dissolved;
The higher the pH value, the stronger the alkalinity of the water, the lower the saturation index, and the less mineral components that can be dissolved.
Heating the water, reducing the water pressure (such as turning on the faucet, etc.), adding chemicals to the water, etc., can cause the pH value to rise. As the pH increases, the ability of water to dissolve mineral components decreases, and these mineral components (mainly calcium carbonate) precipitate out and become scale.
During the operation of circulating systems (such as cooling towers, steam boilers, circulating water treatment systems, etc.), the pressure and temperature are changing for a long time, causing the pH value of the water to rise and become oversaturated.
When calcium and magnesium in hard water are mainly present in the form of bicarbonate [Ca(HCO3)2, Mg(HCO3)2], these salts will decompose when the water is boiled and become basic carbonate precipitates. And remove. Therefore, this hard water is called temporary hard water. If the calcium and magnesium in hard water are mainly in the form of sulfates, chlorides, and nitrates, when the water is boiled, these salts will not precipitate and cannot be removed. This kind of hard water is called permanently hard water.
Calcium and magnesium salts in hard water can interact with sodium stearate, the main ingredient of soap, to generate insoluble calcium or magnesium stearate, which makes part of the soap waste in vain, which is equivalent to reducing the decontamination ability of soap. If the unsoftened hard water is directly injected into the boiler, when the boiler is heated, calcium and magnesium ions will form basic carbonate precipitation, which will accumulate scale on the inner wall and pipes of the boiler, reduce the thermal conductivity of the boiler, and increase the energy In severe cases, it will cause boiler explosion and pipe blockage. Therefore, the boiler water should be softened, and hard water is prone to scum with soap.
Life Notes
Hard water does not pose a direct health hazard. In fact, according to a study by the National Research Council in the United Kingdom, drinking water with hard water is rich in minerals needed by the human body and is an important channel for people to supplement calcium, magnesium and other ingredients. Further research pointed out that when the mineral solubility in water in a certain area is very high, drinking water will become the main source for people to absorb calcium and other components: calcium dissolved in water is the most easily absorbed by the human body.
Soft water, because of its low hardness, has a lighter burden on the baby's body, and is favored by people as the water for brewing milk powder. Because milk powder itself contains nutrients such as protein and minerals, and the brewing of high-hardness hard water will increase the burden on the baby's body. The internal organs of babies are not yet fully developed, and excessive intake of minerals may cause indigestion, so soft water with lower mineral content is more suitable for brewing milk powder.
Hard water has many disadvantages:
1. When reacting with soap, insoluble precipitation is produced, which reduces the washing effect. (This can also be used to distinguish between hard and soft water)
2. Often drinking hard water will increase the incidence of stones in the human filtration system
3. In industry, the precipitation of calcium and magnesium salts will cause boiler scale, hinder heat conduction, and even lead to boiler explosion in severe cases. Due to the problem of hard water, the industry spends tens of millions of yuan every year for the maintenance and replacement of equipment and pipelines.
4. The drinking of hard water will also have a certain impact on human health and daily life. People who do not drink hard water occasionally drink hard water, which will cause gastrointestinal disorders, the so-called "acclimatization"; cooking fish and vegetables with hard water will destroy or reduce the nutritional value of the food because it is not easy to cook; using hard water to make tea will Change the color and aroma of tea and reduce its drinking value; using hard water to make tofu will not only reduce the yield, but also affect the nutritional content of tofu.

Hard water often brings many hazards to people. When hard water is heated, calcium carbonate and magnesium carbonate will precipitate. Since calcium carbonate and magnesium carbonate are insoluble in water, the impact on healthy life is as follows:
1. The mirror surface is covered with water stains;
2. Cotton clothes or towels are hard and stiff, and the color is dull after repeated washing;
3. Water heaters, humidifiers and other equipment pipelines are blocked, flow is reduced, and life is shortened;
4. The white bathtub or toilet and other equipment are yellowed;
5. The faucet and shower head are full of scale, breeding bacteria, and the chrome-plated surface is stained with water;
6. Dry, rough and itchy skin after bathing;
7. If the hair is dry, knotted, and difficult to comb, only special shampoo can be used;
8. The water quality is yellow and there is rust;
9. The formation of boiler scale will hinder the heat transfer of the heater. When the boiler scale is too thick, the boiler will be overheated locally, causing the boiler to rupture and cause accidents, and sometimes it will also cause the boiler to explode;
10. The unpleasant smell of disinfectant is permeated;
11. The ice cubes produced are cloudy in color and have peculiar smell.
China's "Hygienic Standards for Domestic Water" stipulates that the total hardness of water should not be too large. If the hardness is too large, it will have a certain impact on human health and daily life after drinking. If people who do not drink hard water occasionally drink hard water, it will cause gastrointestinal disorders, that is, the so-called "acclimatization", which is what it means. For example, cooking fish and vegetables with hard water often destroys or reduces the nutritional value because it is not easy to cook. Hard water brewing tea will change the color and aroma of the tea and reduce the drinking value. Using hard water to make tofu not only reduces yield, but also affects the nutritional content of tofu. Hard water problems also cost the industry tens of millions of dollars a year to repair and replace equipment and pipelines.
Calcium ions in hard (untreated) water easily form solid calcium carbonate (i.e. limescale), which can bring a lot of trouble to life, such as limescale plaques on water appliances, and reduced washing efficiency with soaps and detergents , After bathing, the skin is rough, the hair is messy and dull, and the washed clothes are dark and stiff. Among them, the damage to the skin and clothing is the most serious.

How can solve the hard water problem? —— Magiko Magnetic Water Softeners/Conditioner.
Our Dailymag Magnetic Magiko employ magnetic water conditioning technology to reverse the polarization of the minerals in your water. This results in the removal of existing corrosion and hard-water scale buildup and is the eco-friendly way to solve your hard water problems and safely enjoy better-tasting, clearer water.
The company that manufactures Dailymag best magnetic water softeners is an organic environmental solutions company that's been around for over 30 years. If you're ready to improve the quality of your water, you should try an Dailymag Magnetic water conditioner.

Magiko is a new brilliant brand of Dailymag Magnetics. It is with excellent design and best quality of magnetic water softeners. We offer a comprehensive magnetic water softeners for you. You will enjoy a unexceptionable experience with Magiko magnetic water softeners and products.
Welcome to inquiry Dailymag Magiko






